Developmental mechanisms specifying tissue-resident macrophages
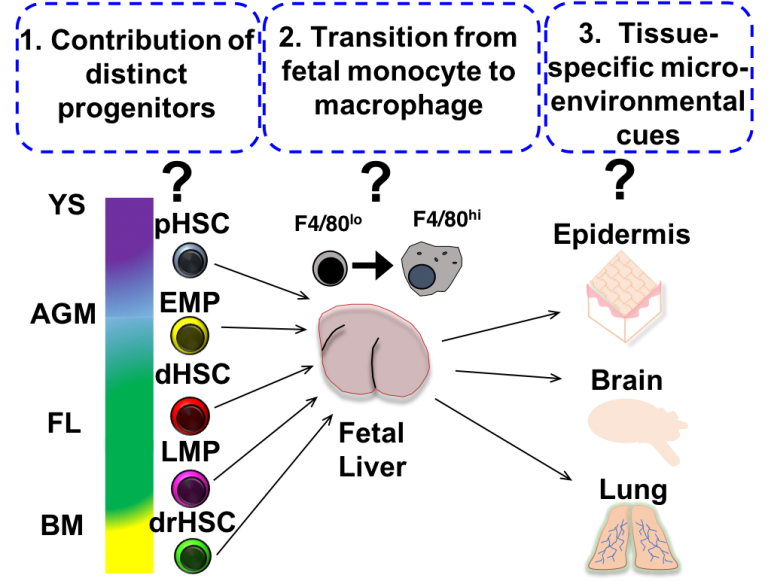 Recent fate-mapping experiments have revealed that the adult macrophage system is “layered”. In addition to classical adult monocyte-derived macrophages, “tissue-resident” macrophages self-maintain across the lifespan through in situ proliferation, independent of contribution from bone marrow-derived monocytes. Lineage tracing experiments have provided direct evidence for a fetal origin of these tissue resident populations in situ, but how distinct waves of hematopoietic cell production during fetal development contribute to adult tissue resident macrophage compartments remains unclear. Furthermore, inadequate understanding of the cellular and molecular mechanisms governing fetal macrophage differentiation has limited dissection of tissue macrophage function and heterogeneity in normal adult tissue homeostasis and disease. The appearance of tissue macrophages so early in ontogeny suggests they both educate and are educated within the local tissue microenvironment across development, both to support normal development and acquire critical tissue homeostatic functions. Our lab is interested in investigating the mechanisms that regulate the development of distinct tissue-resident macrophages across ontogeny in order to gain insight into their function and heterogeneity in development and adult immunity. We have recently identified interleukin-7 (IL-7)signaling as a novel regulator of fetal macrophage development during a very restricted developmental window. We are currently investigating the cellular and molecular events that are regulated by IL-7 signaling during fetal myeloid development using a combination of molecular and genetic approaches in vivo and ex vivo.
Recent fate-mapping experiments have revealed that the adult macrophage system is “layered”. In addition to classical adult monocyte-derived macrophages, “tissue-resident” macrophages self-maintain across the lifespan through in situ proliferation, independent of contribution from bone marrow-derived monocytes. Lineage tracing experiments have provided direct evidence for a fetal origin of these tissue resident populations in situ, but how distinct waves of hematopoietic cell production during fetal development contribute to adult tissue resident macrophage compartments remains unclear. Furthermore, inadequate understanding of the cellular and molecular mechanisms governing fetal macrophage differentiation has limited dissection of tissue macrophage function and heterogeneity in normal adult tissue homeostasis and disease. The appearance of tissue macrophages so early in ontogeny suggests they both educate and are educated within the local tissue microenvironment across development, both to support normal development and acquire critical tissue homeostatic functions. Our lab is interested in investigating the mechanisms that regulate the development of distinct tissue-resident macrophages across ontogeny in order to gain insight into their function and heterogeneity in development and adult immunity. We have recently identified interleukin-7 (IL-7)signaling as a novel regulator of fetal macrophage development during a very restricted developmental window. We are currently investigating the cellular and molecular events that are regulated by IL-7 signaling during fetal myeloid development using a combination of molecular and genetic approaches in vivo and ex vivo.
Influence of maternal inflammation on fetal hematopoietic and immune development
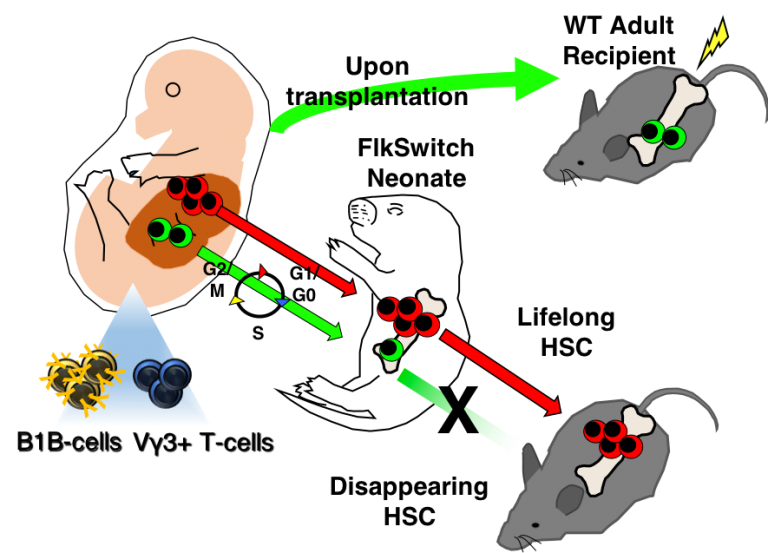 We recently discovered a developmentally-restricted hematopoietic stem cell (drHSC) that only exists during early life but is responsible for producing innate-like immune cells that persist into adulthood (Beaudin et al, Cell Stem Cell, 2016). Innate-like lymphocytes, including B1 B cells, γδ-T cells, and recently described innate lymphoid cells (ILCs), play critical roles in mediating tolerance and rapid response to infection, and have also been implicated in autoimmunity and atopic disorders. The identification of a transient cell of origin that generates a distinct component of adult immunity defines a “critical window” for immune development. A critical developmental window is defined as an early sensitive period during which phenotype can be shaped by intrinsic or extrinsic factors. We hypothesize that developmental perturbation shapes autoimmune disease susceptibility by altering hematopoietic and immune development during a critical window. We have demonstrated that maternal immune perturbation with a single low-dose intraperitoneal injection of poly (i:c) during gestation results in specific expansion drHSCs and their progeny in neonates and abnormal persistence of the drHSC into adulthood. How perturbation of the fetal HSC compartment and establishment of developmentally-restricted immune progeny will affect the functional characteristics of the developing and adult immune system are poorly understood. Currently, our lab uses lineage tracing approaches, gene expression profiling, and ex vivo functional assays to define how the maternal immune response influences immune function across the lifespan. By determining how early life events shape the developing HSC compartment and fetal-derived immune cells, this work will take an innovative approach to identifying the cellular drivers of autoimmune disease susceptibility.
We recently discovered a developmentally-restricted hematopoietic stem cell (drHSC) that only exists during early life but is responsible for producing innate-like immune cells that persist into adulthood (Beaudin et al, Cell Stem Cell, 2016). Innate-like lymphocytes, including B1 B cells, γδ-T cells, and recently described innate lymphoid cells (ILCs), play critical roles in mediating tolerance and rapid response to infection, and have also been implicated in autoimmunity and atopic disorders. The identification of a transient cell of origin that generates a distinct component of adult immunity defines a “critical window” for immune development. A critical developmental window is defined as an early sensitive period during which phenotype can be shaped by intrinsic or extrinsic factors. We hypothesize that developmental perturbation shapes autoimmune disease susceptibility by altering hematopoietic and immune development during a critical window. We have demonstrated that maternal immune perturbation with a single low-dose intraperitoneal injection of poly (i:c) during gestation results in specific expansion drHSCs and their progeny in neonates and abnormal persistence of the drHSC into adulthood. How perturbation of the fetal HSC compartment and establishment of developmentally-restricted immune progeny will affect the functional characteristics of the developing and adult immune system are poorly understood. Currently, our lab uses lineage tracing approaches, gene expression profiling, and ex vivo functional assays to define how the maternal immune response influences immune function across the lifespan. By determining how early life events shape the developing HSC compartment and fetal-derived immune cells, this work will take an innovative approach to identifying the cellular drivers of autoimmune disease susceptibility.
Translation of maternal inflammation by the fetal immune system: effect of congenital toxoplasmosis on fetal hematopoietic and immune development
Previous work by Dr. Beaudin during her postdoc in the Forsberg Lab identified a developmentally-restricted hematopoietic stem cell (drHSC) that exists only during fetal and early neonatal development and specifically gives rise to innate-like lymphocytes that persist across the lifespan and can’t be generated or re-generated in the adult. The existence of a developmentally-limited HSC that contributes to the adult immune system therefore identifies a critical window for the establishment of specific components of neonatal and adult immunity. Interestingly, we have shown that maternal immune activation during pregnancy alters the establishment of drHSCs, leading to persistent postnatal changes to the HSC compartment and increased cellularity of innate-like immune cells in neonates. Innate-like lymphocytes mediate responses to infection and tolerance, and therefore alterations in their establishment have implications for immunizations, atopic disease, and autoimmunity. Our lab has recently begun to model perturbation of fetal immune development in the context of intracellular TORCH pathogen Toxoplasma gondii (T. gondii), a common parasite. During pregnancy, T. gondii can cross the placental barrier and cause spontaneous abortion; in the absence of vertical transmission, maternal inflammation has been linked to fetal resorption and lowered birth weights. Protective immunity may be generated in surviving offspring at the expense of fetal wasting but the mechanisms that underlie this interaction are not well understood. Our ultimate goal is to link the effects of congenital toxoplasmosis on the developing immune system with altered disease susceptibility in later life. Using a combination of in-vivo reporter lines, genetic approaches, and genome-wide expression analysis, we aim to characterize changes to the fetal hematopioetic compartment and at the fetal hematopoietic and placental interface of infected versus non-infected pregnancies. We will then trace the outcome of these infections on the neonatal and adult hematopoietic and immune populations to determine lasting perturbations to the mature immune system. Functional assays, such as susceptibility to toxoplasma infection and allergic airway inflammation will be used to determine altered disease susceptibility.

